Ethide Laboratories - USP 88 In-Vivo Cytotoxicity Testing
Por um escritor misterioso
Descrição
Learn what USP 88 cytotoxicity tests are available and which ones you will need to meet the regulatory requirements for your medical devices.

Drug-mediated toxicity: illuminating the 'bad' in the test tube by means of cellular assays?: Trends in Pharmacological Sciences

Championing In Vitro Biocompatibility/Biological Reactivity Testing for Plastics Used in Pharma Manufacturing

Media and Supplements for In Vitro Hematotoxicity Testing
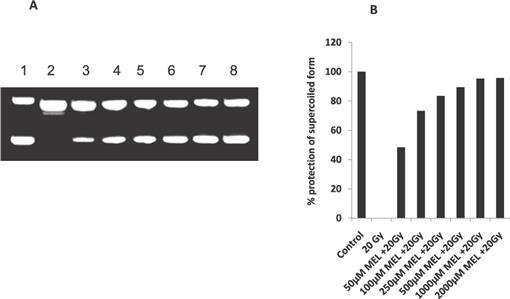
Sesamol as a Potential Radioprotective Agent: In Vitro Studies

EP2897610B1 - Pkc delta inhibitors for use as therapeutics - Google Patents

Cytotoxicity Test
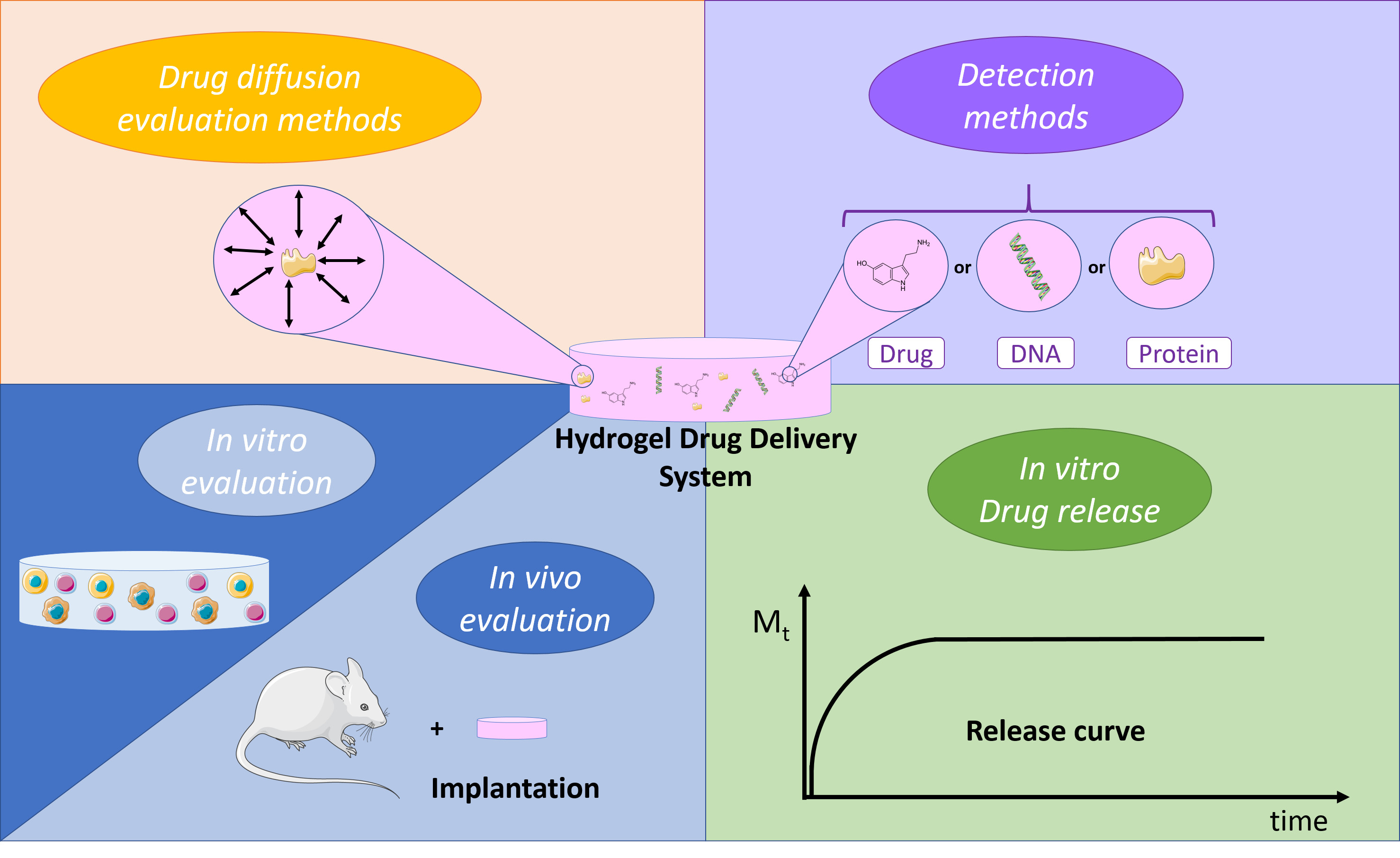
Pharmaceutics, Free Full-Text
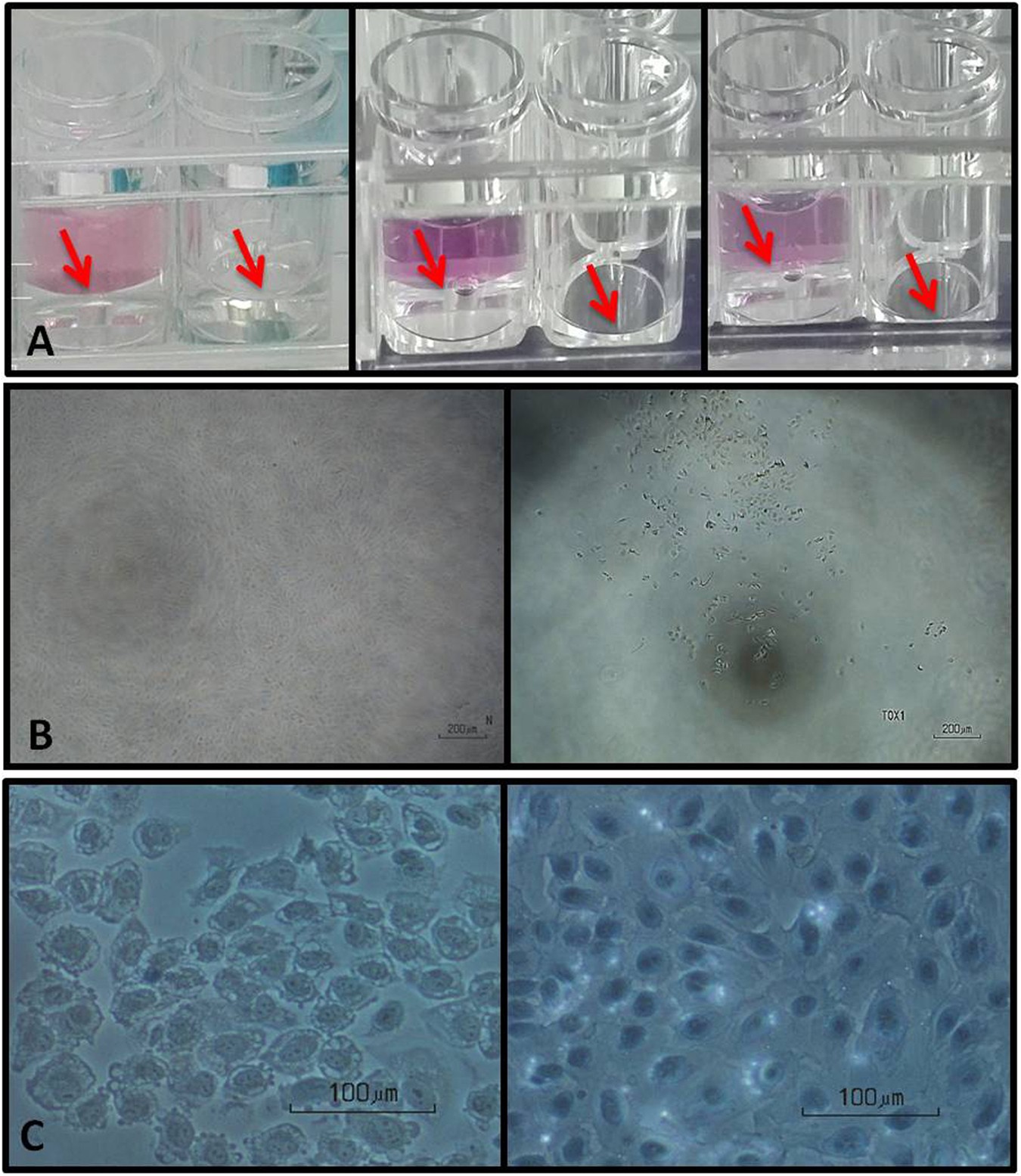
Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation

Cancer Therapy Volume 2 Issue B by Cancer Therapy - Issuu
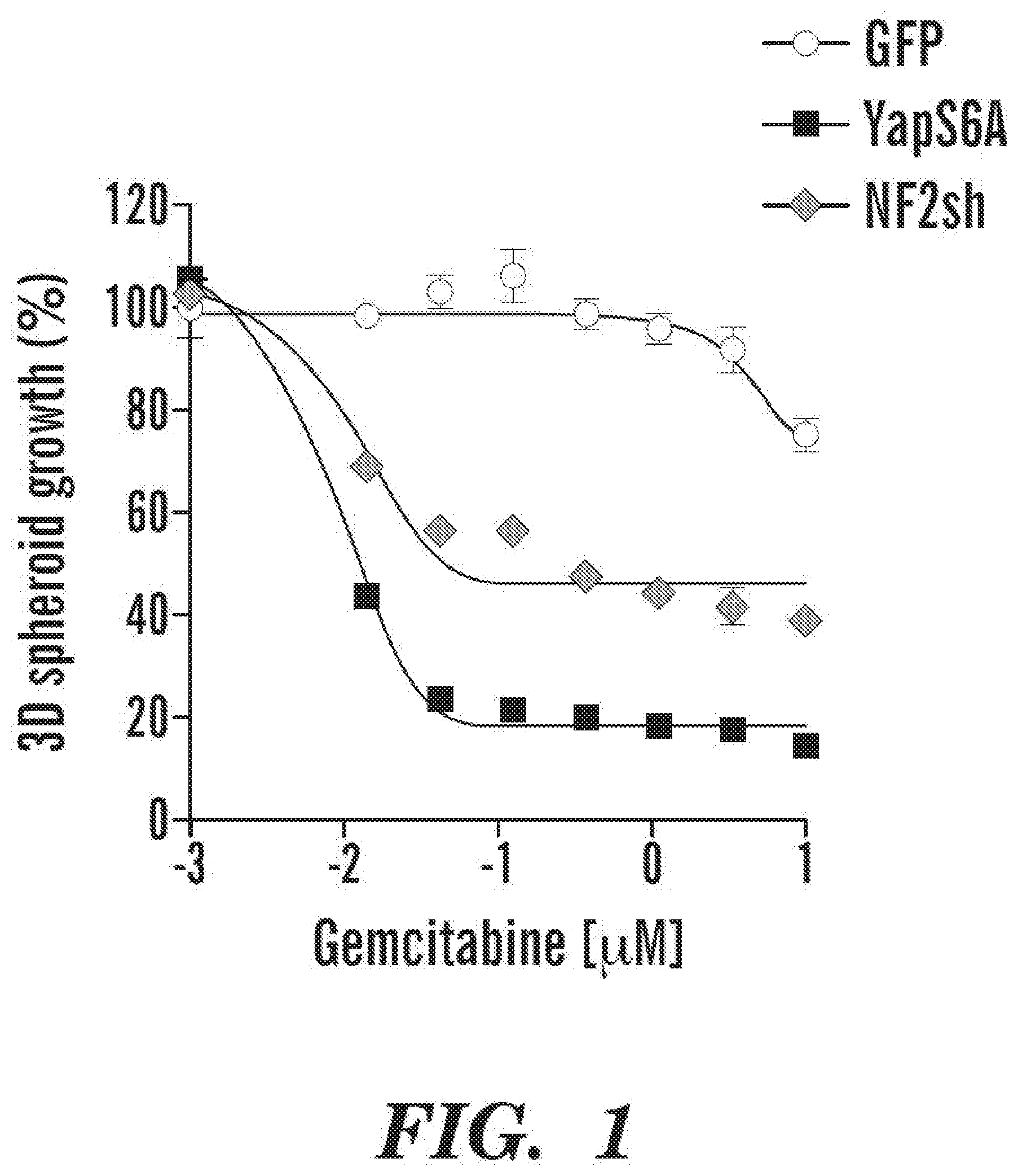
Methods And Compositions Relating To The Diagnosis And Treatment Of Cancer GUJRAL; Taran ; et al. [PRESIDENT AND FELLOWS OF HARVARD COLLEGE]
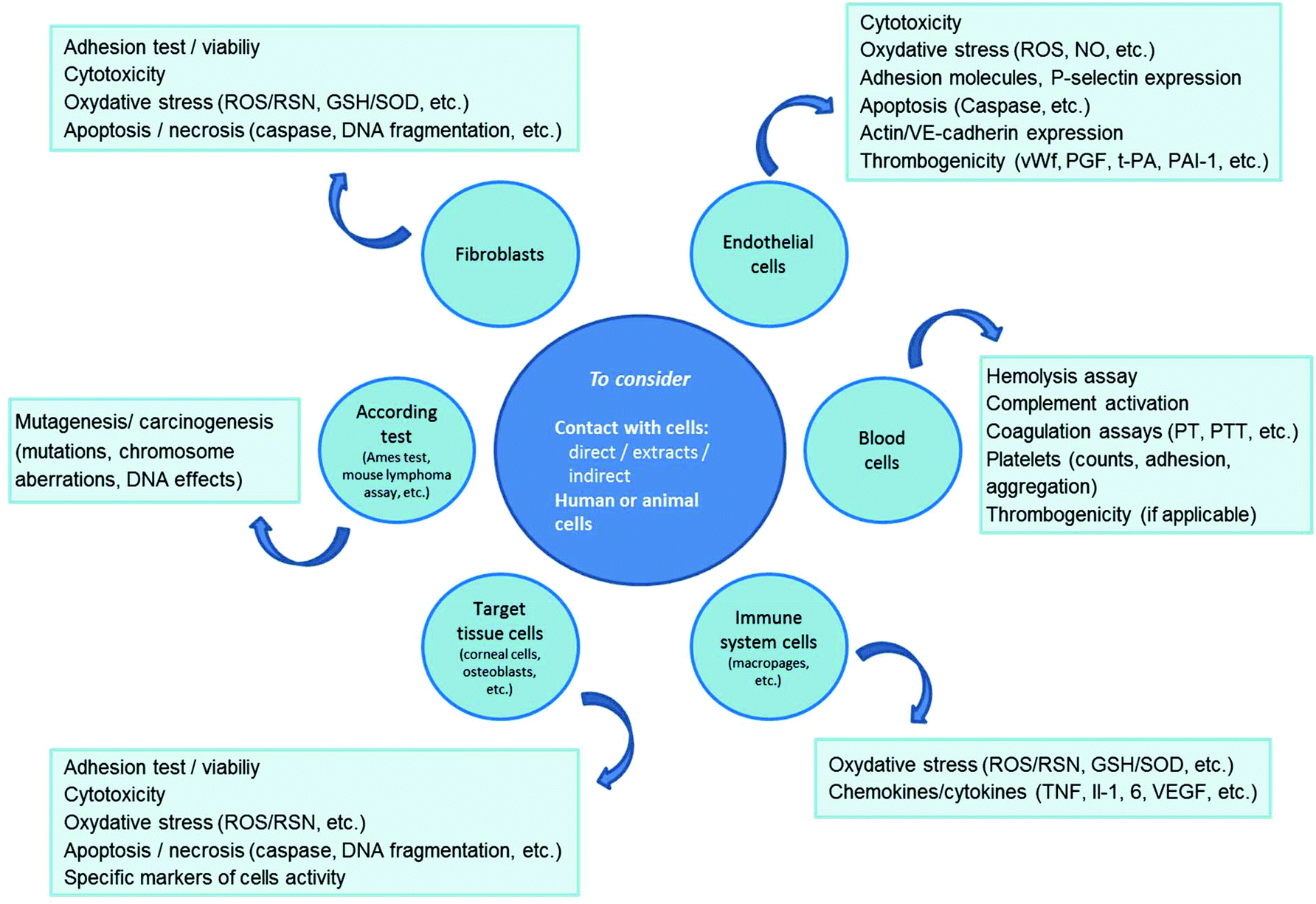
Biocompatibility of polymer-based biomaterials and medical devices – regulations, in vitro screening and risk-management - Biomaterials Science (RSC Publishing) DOI:10.1039/C8BM00518D

Proteasomal Degradation of TRAF2 Mediates Mitochondrial Dysfunction in Doxorubicin-Cardiomyopathy

Redox Signaling by Reactive Electrophiles and Oxidants. - Abstract - Europe PMC
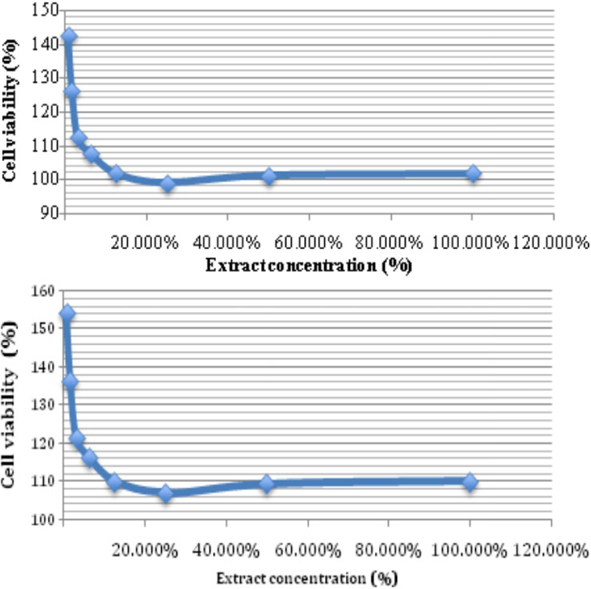
In vitro and in vivo biocompatibility of Ti-6Al-4V titanium alloy and UHMWPE polymer for total hip replacement

Doxorubicin-loaded pH-responsive nanoparticles coated with chlorin e6 for drug delivery and synergetic chemo-photodynamic therapy - IOPscience
de
por adulto (o preço varia de acordo com o tamanho do grupo)







