FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Descrição
Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

Tool to easily search for a stem cell clinical trial for your

Systematic Review of Induced Pluripotent Stem Cell Technology as a

FDA Approves 2 New Bispecifics for Relapsed or Refractory Multiple
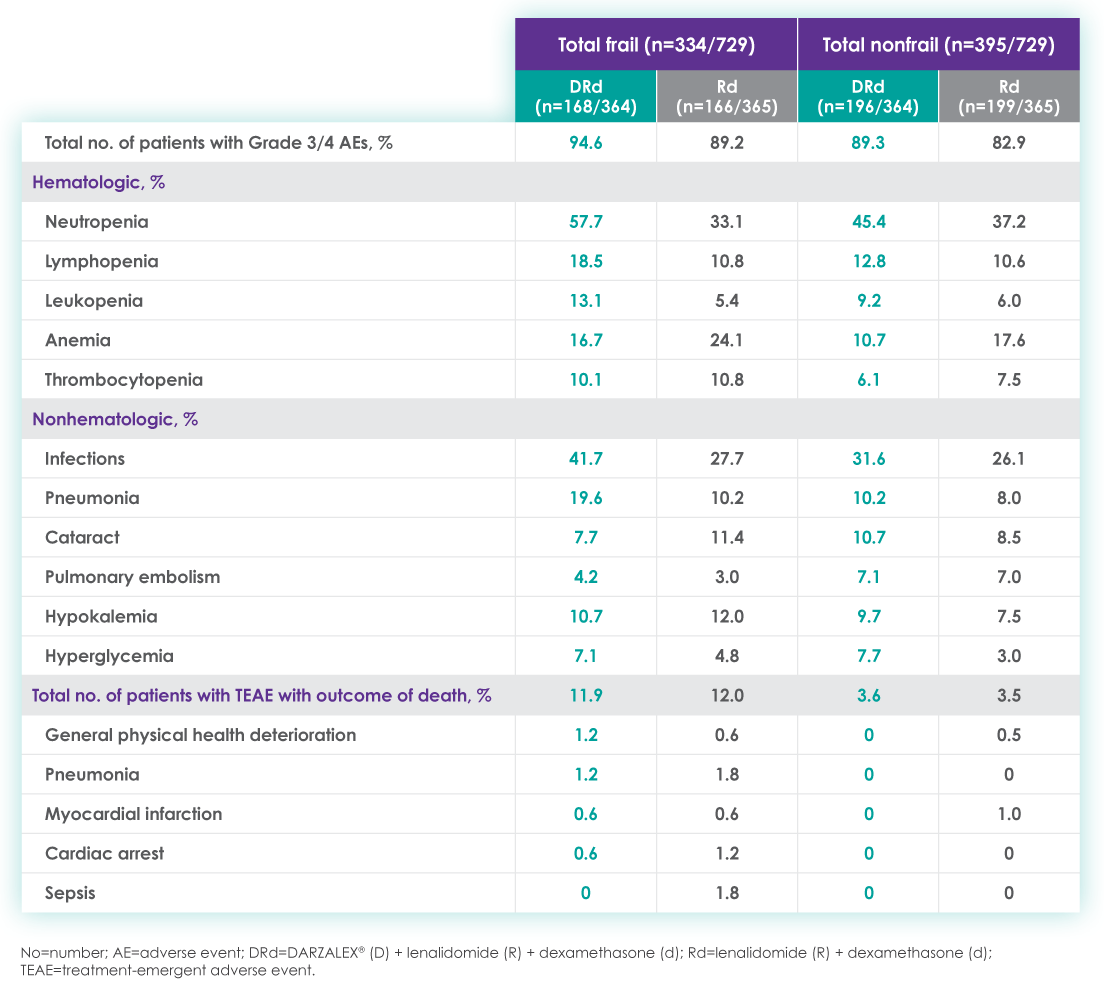
One Expert's Approach in Transplant-Ineligible, Newly Diagnosed

Adult Acute Lymphoblastic Leukemia Treatment (PDQ®) - PDQ Cancer

FDA Approves First CRISPR/Cas9 Gene-Editing Therapy

FDA approves cell therapy for patients with blood cancers to

Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed

New FDA approved stem cell therapy trial for repair of knee
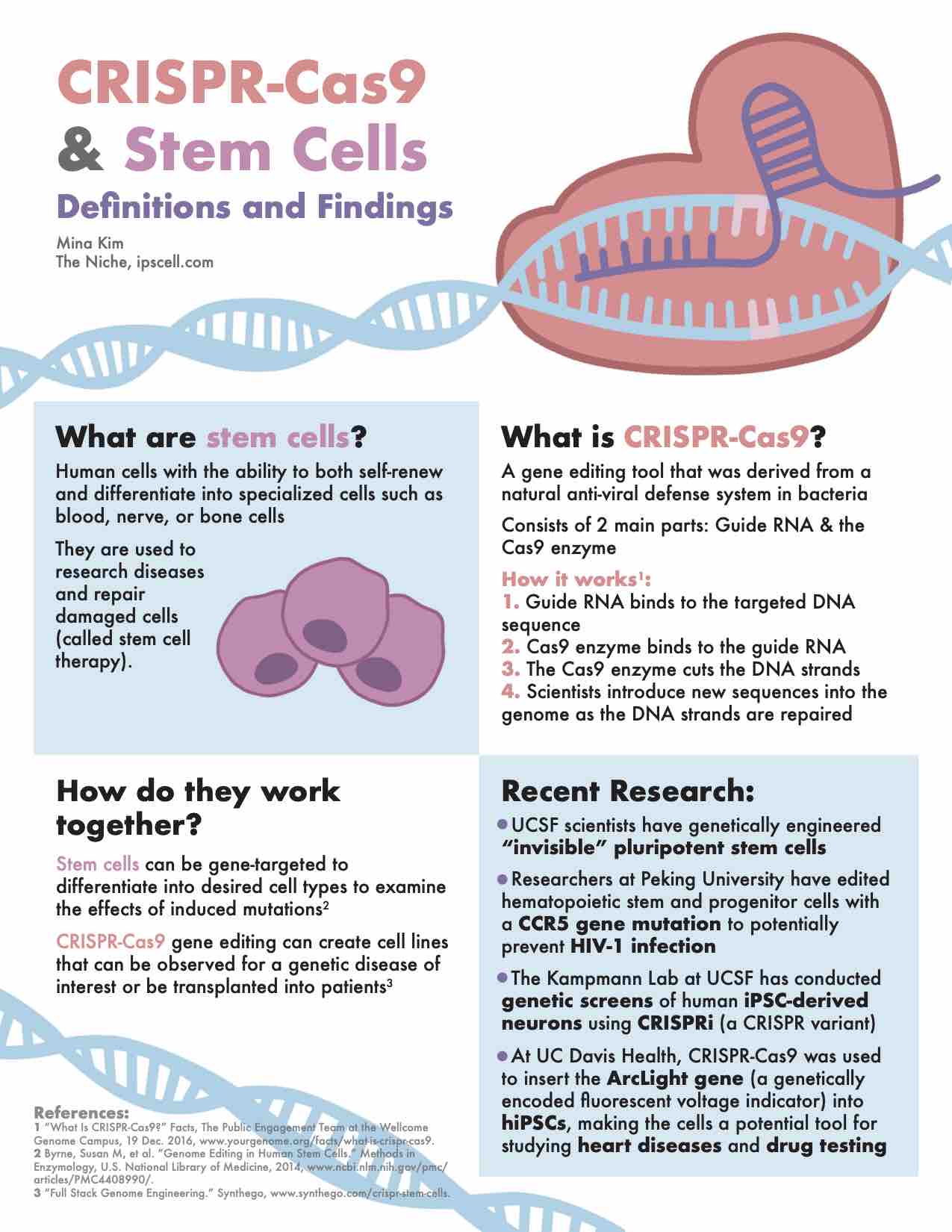
New research on CRISPR gene-editing in stem cells, infographic
de
por adulto (o preço varia de acordo com o tamanho do grupo)



:max_bytes(150000):strip_icc()/k53KU-s-p-500-biggest-gains-and-losses39-d32461681cb845129e5382eb74d060a6.png)