What will be the shape of ICl 2 among the following?A. BentB. Trigonal planarC. LinearD. Trigonal bipyramidal
Por um escritor misterioso
Descrição
What will be the shape of ICl 2 among the following?A. BentB. Trigonal planarC. LinearD. Trigonal bipyramidal
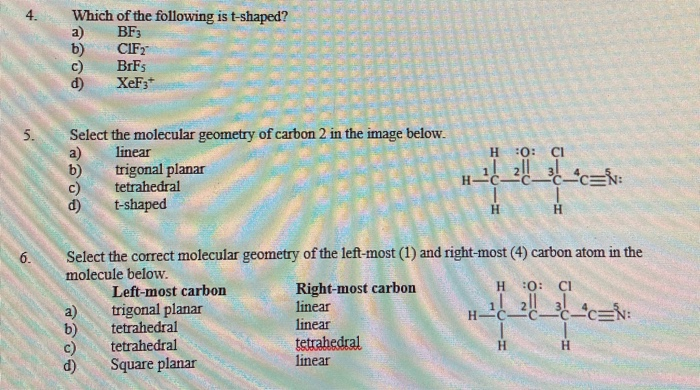
Solved 2 bent The molecular structure of the C102 ion is

Which of the following arrangements of lone pairs and bonding

Answered: Determine the electron geometry (eg)…
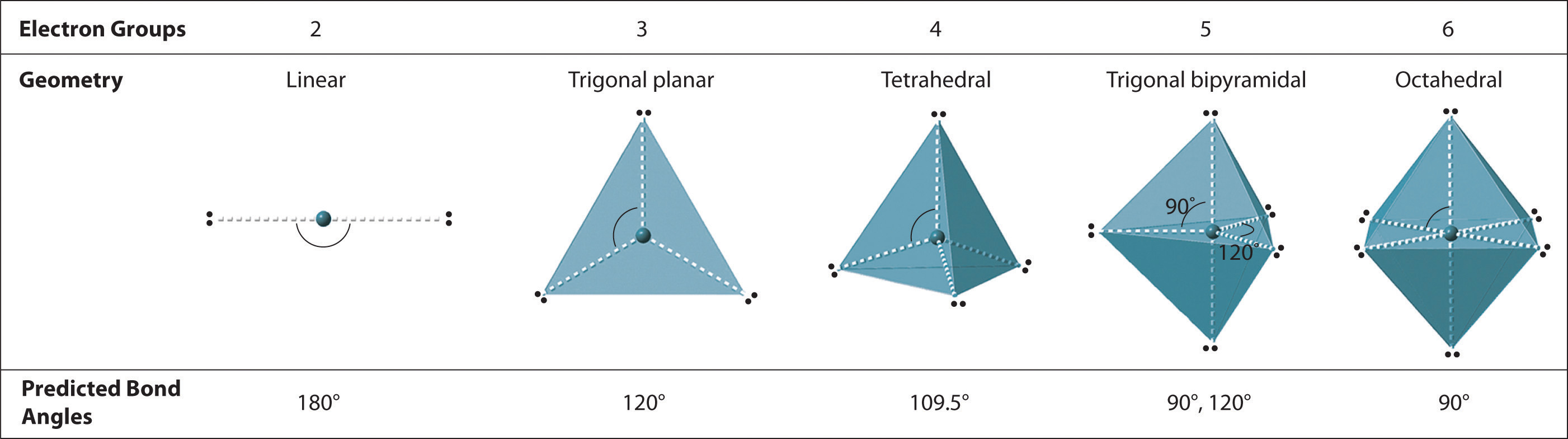
VSEPR

What will be the shape of ICl 2 among the following?A. BentB

Shapes of Molecules

VSEPR Theory: Explanation, Chart, and Examples

What will be the shape of ICl 2 among the following?A. BentB

According to VSEPR theory, the shape of ICl_4^- is described as
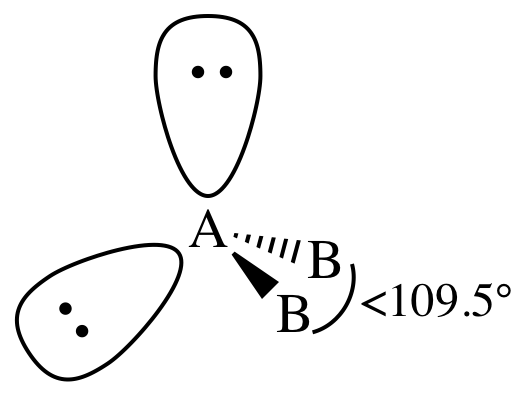
Section 11-1: Molecular Geometry: Using VSEPR Theory to

According to VSEPR theory, in which fashion will the bonds and

Calaméo - Test Bank For Chemistry A Molecular Approach 2nd Edition
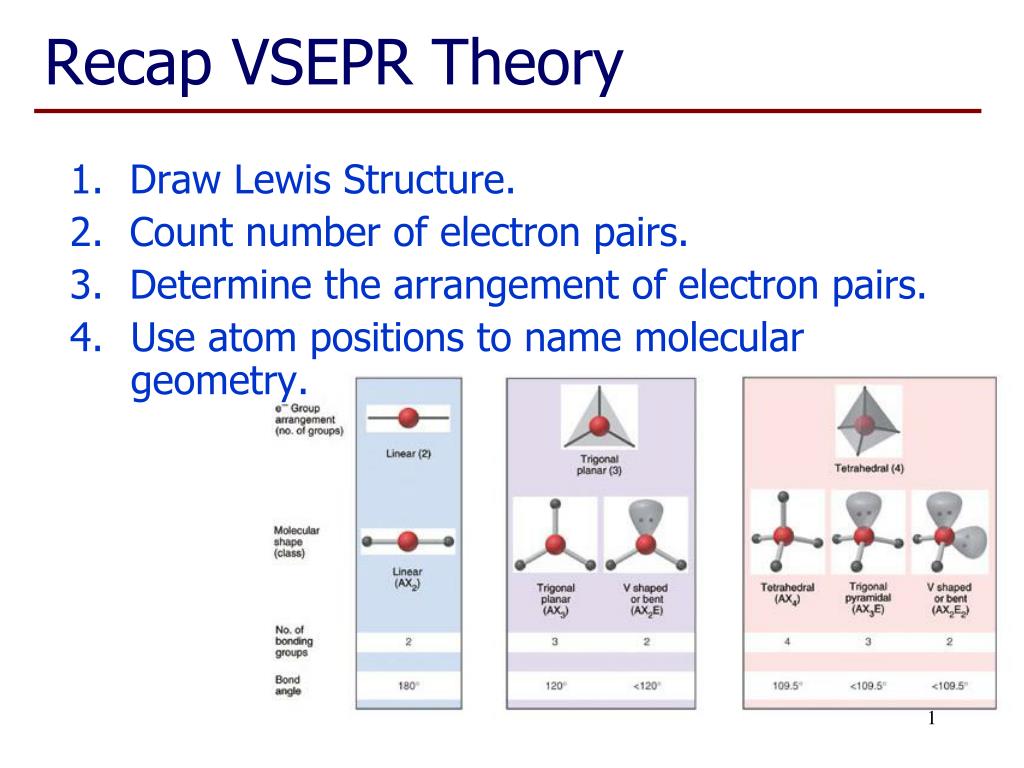
PPT - Recap VSEPR Theory PowerPoint Presentation, free download
de
por adulto (o preço varia de acordo com o tamanho do grupo)