New approach methodologies in human regulatory toxicology – Not if, but how and when! - ScienceDirect
Por um escritor misterioso
Descrição

Regulatory assessment of chemical mixtures: Requirements, current approaches and future perspectives - ScienceDirect

Regulatory Toxicology and Pharmacology 44 - TRIPHASE Pharma

Human risk associated with exposure to mixtures of antiandrogenic chemicals evaluated using in vitro hazard and human biomonitoring data - ScienceDirect
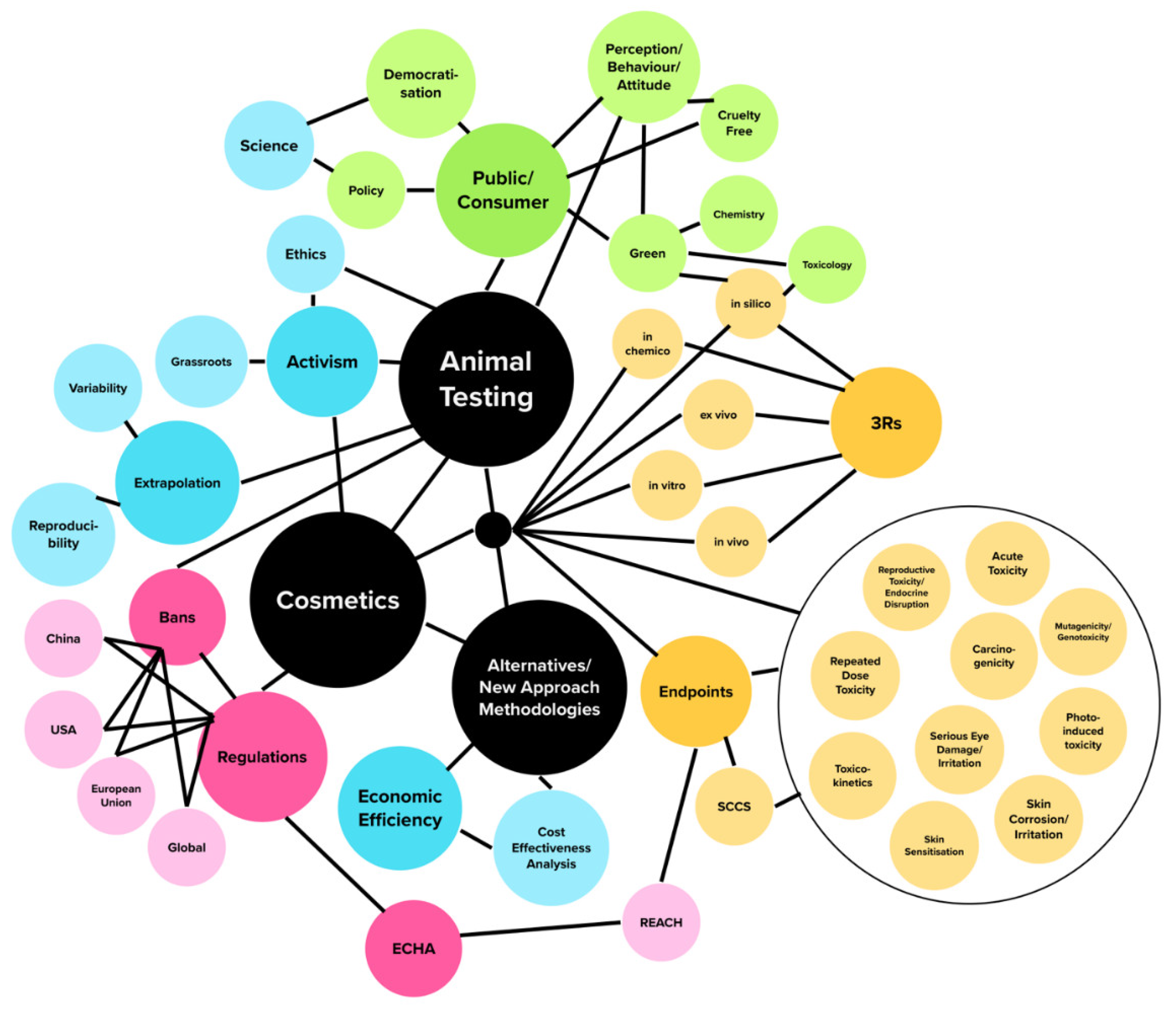
Cosmetics, Free Full-Text

Sustainability, Free Full-Text

Regulatory Toxicology and Pharmacology, Vol 137, January 2023

Dose Response Modeling - an overview
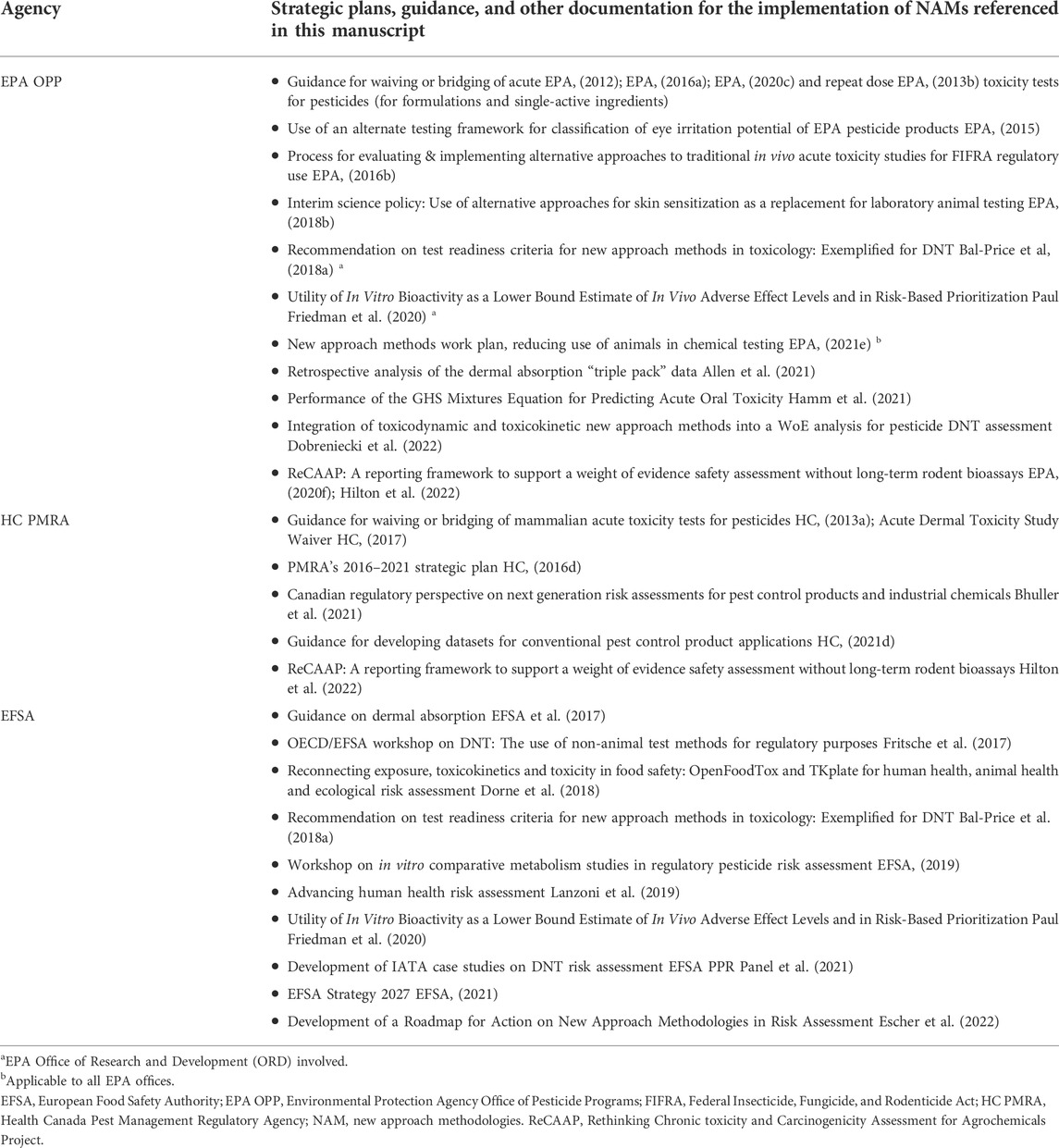
Frontiers Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health

New approach methodologies for exposure science - ScienceDirect
Approach to toxicity testing suggested by the NRC (USA) A. Tox c ty
de
por adulto (o preço varia de acordo com o tamanho do grupo)







